H. Cabral, H. Kinoh, K. Kataoka
Acc. Chem. Res. 53 Issue 12 2765-2776 (2020) (DOI: 10.1021/acs.accounts.0c00518)
Tumor-targeted nanomedicine for immunotherapy
Abstract:
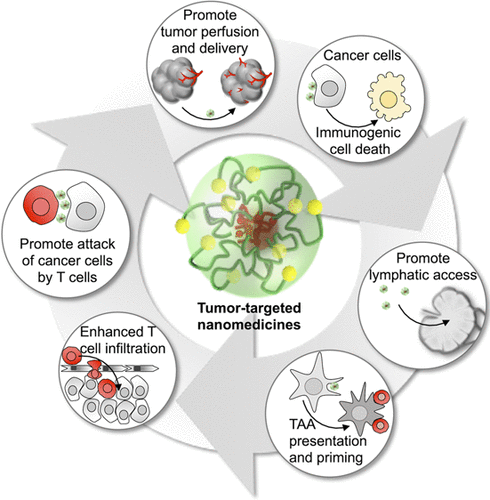
Therapeutic manipulation of the immune system against cancer has revolutionized the treatment of several advanced-stage tumors. While many have benefited from these treatments, the proportion of patients responding to immunotherapies is still low. Nanomedicines have promise to revolutionize tumor treatments through spatiotemporal control of drug activity. Such control of drug function could allow enhanced therapeutic actions of immunotherapies and reduced side effects. However, only a handful of formulations have been able to reach human clinical studies so far, and even fewer systems are being used in the clinic. Among translatable formulations, self-assembled nanomedicines have shown unique and versatile features for dealing with the heterogeneity and malignancy of tumors in the clinic. Such nanomedicines can be designed to promote antitumor immune responses through a series of immunopotentiating functions after being directly injected into tumors, or achieving selective tumor accumulation upon intravenous administration. Thus, tumor-targeted nanomedicines can enhance antitumor immunity by several mechanisms, such as inducing immunogenic damage to cancer cells, altering the tumor immune microenvironment by delivering immunomodulators, or eliminating or reprogramming immunosuppressive cells, enhancing the exposure of tumor-associated antigens to antigen presenting cells, stimulating innate immunity mechanisms, and facilitating the infiltration of antitumor immune cells and their interaction with cancer cells. Moreover, nanomedicines can be engineered to sense intratumoral stimuli for activating specific immune responses or installed with ligands for increasing drug levels in tumors, granting subcellular delivery, and triggering immune signals and proliferation of immune cells. Thus, the ability of nanomedicines to exert immunomodulatory functions selectively in tumor and tumor-associated tissues, such as draining lymph nodes, increases the efficiency of the treatments, while avoiding systemic immunosuppressive toxicities and the exacerbation of adverse immune responses. Moreover, the compartmentalized structure of self-assembled nanomedicines offers the possibility to coload a variety of drugs for controlled pharmacokinetics, enhanced tumor delivery, and synergistic therapeutic output. Also, by integrating imaging functionalities into nanomedicines, it is possible to develop theranostic platforms reporting the immune settings of tumors as well as the effects of nanomedicines on the tumor immune microenvironment. Herein, we critically reviewed significant strategies for developing nanomedicines capable of potentiating antitumor immune responses by surmounting biological barriers and modulating antitumor immune signals. Moreover, the potential of these nanomedicines for developing innovative anticancer treatments by targeting particular cells is discussed. Finally, we present our perspectives on the awaiting challenges and future directions of nanomedicines in the age of immunotherapy.